Indication
HYAJOINT Plus synovial Fluid Supplement is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacological therapy or simple analgesics.
Therapeutic efficacy
HYAJOINT Plus cushions the joint from external mechanical forces and protects the joint and synovial tissues.
Important Safety Information for HYAJOINT Plus
You should not receive HYAJOINT Plus if you have an infection in your knee or in the skin around your knee. Before receiving HYAJOINT Plus injection, tell your health professional if you have had an allergic symptoms before, such as flushing of the face, scratc1hy throat, pain or tightness in the chest, rash, itching or hives to any hyaluronic acid based products. The injection of HYAJOINT Plus can only be performed by a qualified health care providers. HYAJOINT Plus has not been tested to show pain relief in joints other than the knee.
If you are taking blood thinners such as aspirin or warfarin, you may need to stop them for a few days before your knee injection. You should always discuss this with your doctor first.
Products shown may not be available in all countries or may be known by a different name. Indications for use may vary from country to country. Therefore, please always refer to the product information approved in your country.

Use in the Elderly
Careful attention should be paid to the decline of the organ function in the elderly.

Usage in Pregnancy and Lactation
If the benefits of treatment outweigh the risk of adverse effects. Breast feeding should be ceased during the treatment period.

Usage in children
The safety of use in children has not been established so treating practitioners will need balance the risk of adverse events against the benefits of treatment.
Possible Side Effects of a HYAJOINT Plus Injection
HYAJOINT Plus injections when done by a trained practitioner are generally very safe, however like all medications there are some potential side effects which must be considered before deciding to go ahead with an injection.
Some people may experience initial irritations such as tenderness, redness and swelling after injection, this may last for several days, depending on the individual and the severity of osteoarthritis. The patient also may feel sensations of tingling or numbness. If such side effects do not go away after a reasonable amount of time, contact the orthopedic physician’s office to determine if an evaluation is warranted.
The injection is often given with some local anesthetic, once the anesthetic has worn off there may be some post injection pain, which usually lasts no more than 48 hours.
HYAJOINT Plus, A LONG TERM TREATMENT FOR SYMPTOMATIC OSTEOARTHRITIS WITH A SINGLE INJECTION FOR PAIN RELIEF AND RESTORING MOBILITY1.
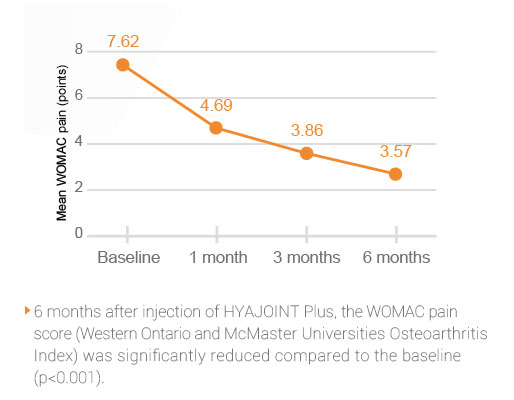
In a prospective study1, HYAJOINT PlusTM relieved pain in patients with symptomatic knee OA for up to 6 months following a single course treatment1. HYAJOINT Plus is both safe and effective for the treatment of knee OA.
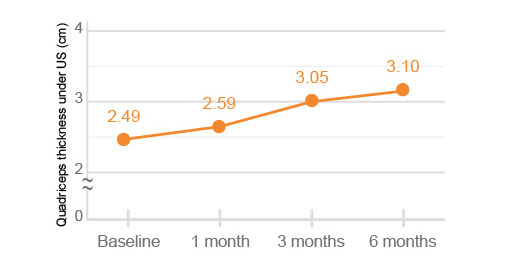
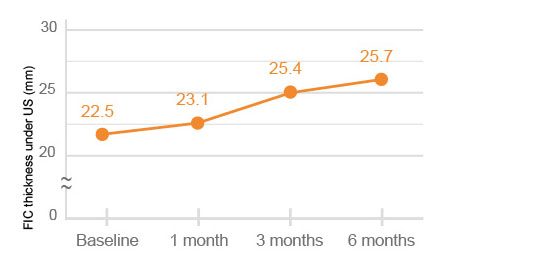
The quadriceps thickness and FIC (femoral intercondylar cartilage) thickness under US (ultrasonography) were significantly improved at 6 months after HYAJOINT PlusTM injection compared to the baseline (p<0.001).
Safety data: No infections, allergies or other serious adverse events were reported.
Effectiveness of HYAJOINT Plus in a prospective, randomized, controlled, double-blind trial of Safety and Efficacy2.
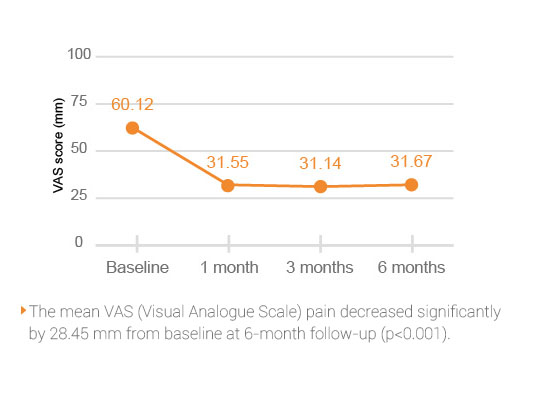
HYAJOINT Plus is superior to S brand in terms of reducing the VAS(Visual Analogue Scale) pain score at 1, 3, and 6 months and the WOMAC stiffness score at 6 months.
Better improvement of pain relief by 33.3 mm on VAS score in patients treated with HYAJOINT Plus compared to 23.4 mm in the S brand group at 6 months (mean difference between groups -6.6; p=0.045)2.
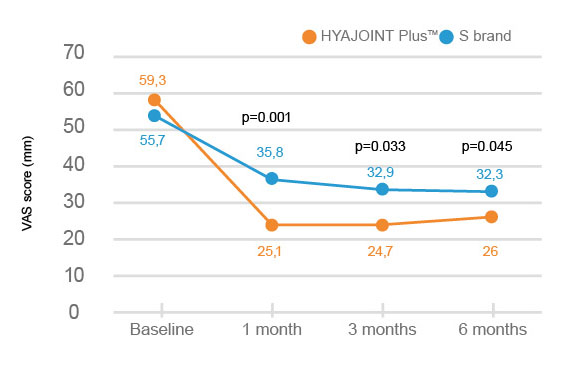
The frequencies and types of adverse events were comparable between S brand and HYAJOINT Plus groups. The majority of adverse events were mild or moderate, lasted 1 to 3 days, and resolved
spontaneously or responded well to simple analgesics. N1o allergies, pseudosepsis or serious adverse events occurred during the study in either group2.
References
1. Tuan S., Liou I., Su H., Tsai Y., Chen G. and Sun S. Improvement of self-reported functional scores and thickening of quadriceps and femoral intercondylar cartilage under ultrasonography after single intra-articular injection of a novel cross-linked hyaluronic acid in the treatm1ent of the knee osteoarthritis. JBMR. 2018 ; 31 : 709-718
2. Sun, S.F., Hsu, C.W., Lin, H.S., Liou I.H., Chen, Y.H. and Hung C.L. Comparison of Single Intra-Articular Injection of Novel Hyaluronan (HYA-JOINT Plus) with Synvisc-One for Knee Osteoarthritis. JBJS. 2017 ; 99(6) : 462-471
Hong Kong Distributor:
LEMAN HONSON PHARMACEUTICAL SPECIALIST COMPANY LIMITED
FLAT/RM H9, 23/F SUPERLUCK INDUSTRIAL CENTRE PHASE 2, 57 SHA TSUI ROAD, TSUEN WAN, NT
Tel No. +852 5402 8585


